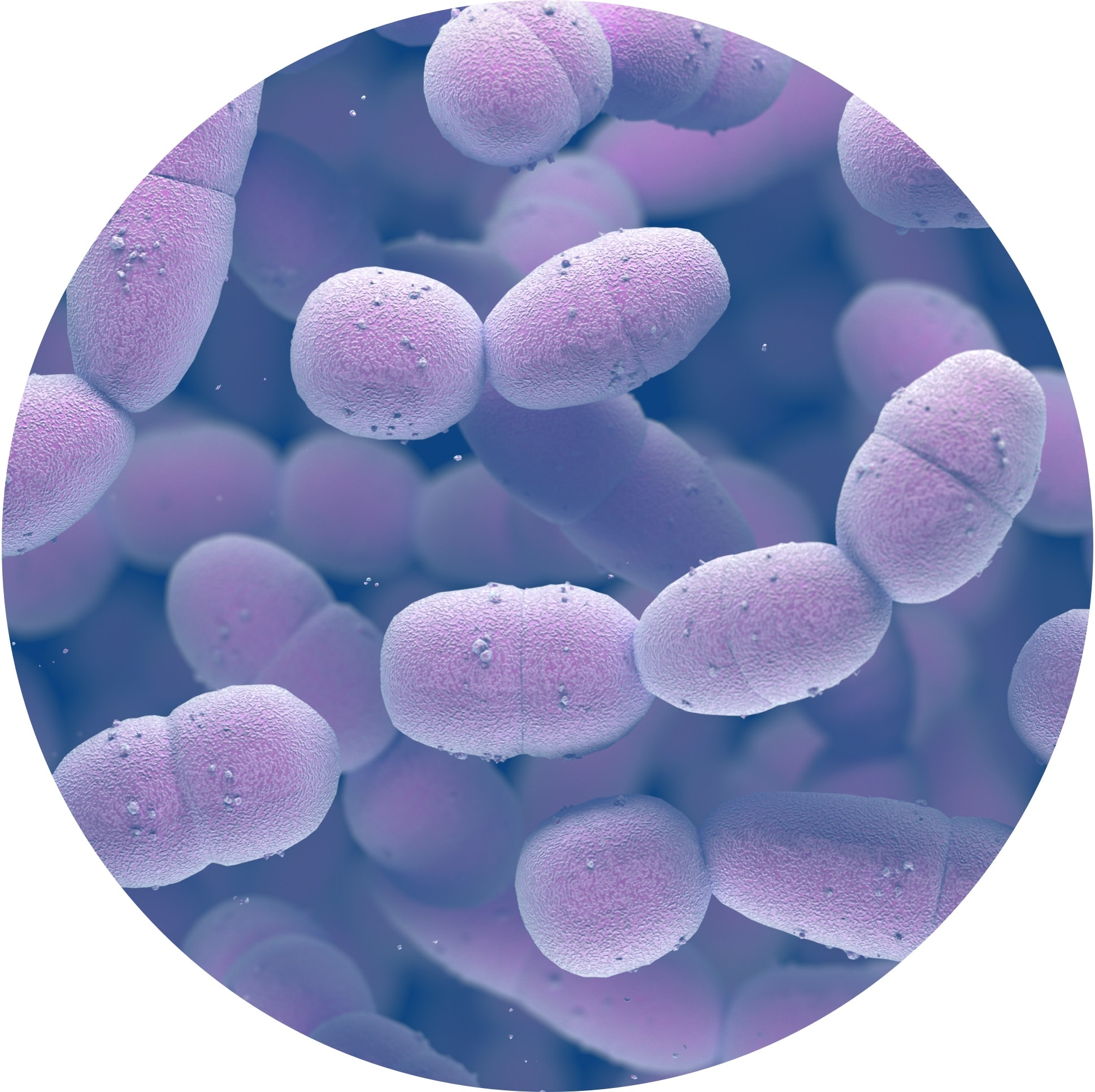
Impact of Serotypes on Invasive Pneumococcal Disease
Polysaccharide Capsule:
Primary Virulence Factor1–5

find out more
IgA=immunoglobulin A; Ply=pneumolysin.
Serotypes Responsible for Majority of
Pneumococcal Disease1–8
There are approximately 100 known pneumococcal serotypes.2 However, most pneumococcal disease worldwide is caused
by certain serotypes (highlighted here).3–7
Study designs vary; data cannot be used to form conclusions about the global burden of disease.
Serotypes Responsible for
Burden of Pneumococcal Disease1–8
Serotypes

United States3,a
Serotypes

Brazil7,e
Serotypes

Europe4,b
Serotypes

Tunisia5,c
Serogroup/Serotype

China6,d
Study designs vary. Data are representative epidemiological data.
Serotypes Responsible for Burden of Invasive
Pneumococcal Disease, United States1–4

to find out
more
IPD=invasive pneumococcal disease.
Indications and Usage
- PNEUMOVAX 23 is a vaccine indicated for active immunization for the prevention of pneumococcal disease caused by the 23 serotypes contained in the vaccine (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, and 33F).
- PNEUMOVAX 23 is approved for use in persons 50 years of age or older and persons aged ≥2 years who are at increased risk for pneumococcal disease.
- PNEUMOVAX 23 will not prevent disease caused by capsular types of pneumococcus other than those contained in the vaccine.
Select Safety Information
- Do not administer PNEUMOVAX 23 to individuals with a history of a hypersensitivity reaction to any component of the vaccine.
- Defer vaccination with PNEUMOVAX 23 in persons with moderate or severe acute illness.
- Use caution and appropriate care in administering PNEUMOVAX 23 to individuals with severely compromised cardiovascular and/or pulmonary function in whom a systemic reaction would pose a significant risk.
PNEUMOVAX®23
(Pneumococcal Vaccine Polyvalent)
The Serotypes in PNEUMOVAX®23 (Pneumococcal Vaccine Polyvalent)
Account for the Majority of Invasive Pneumococcal Disease Cases
in Adults, United States, 20181,a
The figure depicts CDC epidemiological data and does not reflect the vaccine efficacy.
PNEUMOVAX 23 will not prevent disease caused by capsular types of pneumococcus other than those contained in the vaccine.
CDC=Centers for Disease Control and Prevention; IPD=invasive pneumococcal disease.
Select Safety Information for PNEUMOVAX®23
(Pneumococcal Vaccine Polyvalent)
- Available human data from clinical trials of PNEUMOVAX 23 in pregnancy have not established the presence or absence of a vaccine-associated risk.
- Since elderly individuals may not tolerate medical interventions as well as younger individuals, a higher frequency and/or a greater severity of reactions in some older individuals cannot be ruled out.
Of the Top 10 Most Common Serotypes Causing IPD in
Adults Aged ≥65 Years, 6 are Included in PNEUMOVAX®23
(Pneumococcal Vaccine Polyvalent)1,a
included in PNEUMOVAX 23
PNEUMOVAX 23 will not prevent disease caused by capsular types of pneumococcus other than those contained in the vaccine.
IPD=invasive pneumococcal disease.
Of the Top 10 Most Common Serotypes Causing IPD in
Adults Aged ≥65 Years, 6 are Included in PNEUMOVAX®23
(Pneumococcal Vaccine Polyvalent)1,a
included in
PNEUMOVAX 23
PNEUMOVAX 23 will not prevent disease caused by capsular types of pneumococcus other than those contained in the vaccine.
IPD=invasive pneumococcal disease.
Select Safety Information for PNEUMOVAX®23
(Pneumococcal Vaccine Polyvalent)
- Persons who are immunocompromised, including persons receiving immunosuppressive therapy, may have a diminished immune response to PNEUMOVAX 23.
- PNEUMOVAX 23 may not be effective in preventing pneumococcal meningitis in patients who have chronic cerebrospinal fluid (CSF) leakage resulting from congenital lesions, skull fractures, or neurosurgical procedures.

health care professionals.
Summary1-6
- Although there are ~100 known pneumococcal serotypes, a smaller number of serotypes are responsible for the majority of invasive pneumococcal disease.1,2
- Serotypes differ in their prevalence and tendency to cause invasive disease (eg, by patient age group, geographic area, site of infection).2,3
- Serotypes 3 and 22F are among the most common serotypes causing invasive pneumococcal disease in the overall US population.4
- Of the top 10 most common serotypes causing IPD in adults ≥65 years, 6 (3, 22F, 11A, 9N, 33F, and 19A) are included in PNEUMOVAX®23 (Pneumococcal Vaccine Polyvalent).5
- Among US adults, approximately 72% of IPD cases in those aged 19–64 and approximately 57% of IPD cases in those aged ≥65 are caused by serotypes included in PNEUMOVAX 23.6
- PNEUMOVAX 23 will not prevent disease caused by capsular types of pneumococcus other than those contained in the vaccine.
Select Safety Information for PNEUMOVAX®23
(Pneumococcal Vaccine Polyvalent)
- The most common adverse reactions, reported in >10% of subjects vaccinated with PNEUMOVAX 23 for the first time in a clinical trial, were: injection-site pain/soreness/tenderness, injection-site swelling/induration, headache, injection-site erythema, asthenia and fatigue, and myalgia.
- For subjects aged 65 years or older in a clinical study, systemic adverse reactions which were determined by the investigator to be vaccine-related were higher following revaccination than following initial vaccination.
- Vaccination with PNEUMOVAX 23 may not offer 100% protection from pneumococcal infection.

health care professionals.
Survey
Thank you for your response
Before administering
PNEUMOVAX®23 (Pneumococcal Vaccine Polyvalent),
please read the accompanying Prescribing Information.
The Patient Information also is available.

Copyright © 2021 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved. US-PNX-01289 07/21